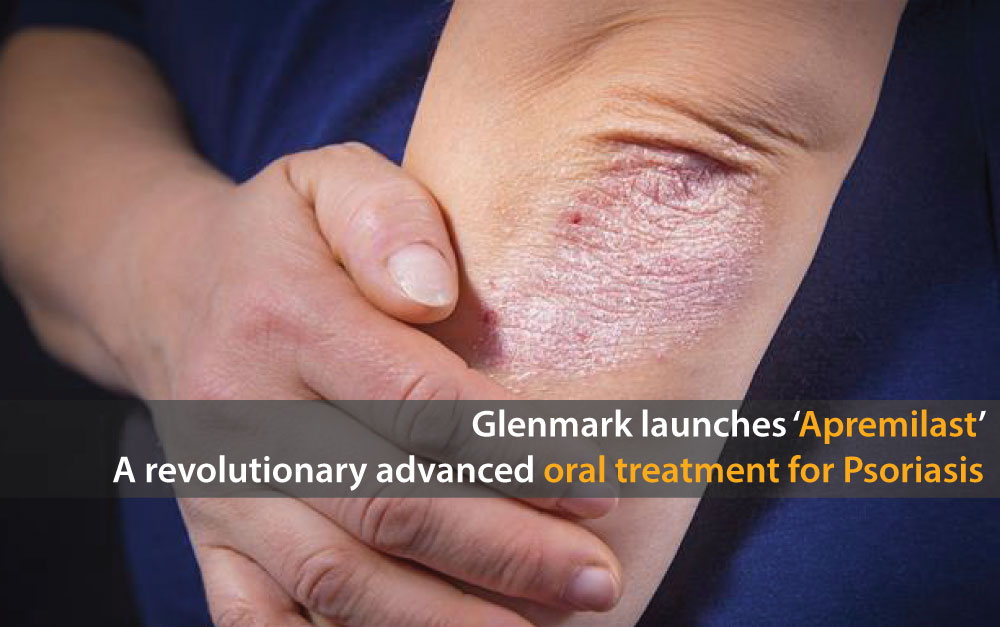
Glenmark Launches ‘Apremilast’ – A Revolutionary Advanced Oral Treatment for Psoriasis
Globally, about 3% of the world population has some form of psoriasis. Another study reveals that the prevalence of psoriasis in countries ranges between 0.09% and 11.43%, making psoriasis as one of the serious issues.
Glenmark Pharmaceuticals Limited, a research-led global integrated pharmaceutical company which has been in the field of dermatology for more than four decades, now has announced the launch of Apremilast under the brand name ‘APREZO’, the first advanced Oral Systemic treatment for psoriasis in India which is also DCGI approved. Apremilast is a phosphodiesterase4 (PDE4) inhibitor which is indicated for the treatment of moderate to severe psoriasis. The launch of Apremilast will revolutionize the treatment of psoriasis impacting close to 33 million Indians suffering from the condition.
Apremilast is an advanced oral treatment for psoriasis which addresses the limitations of the current available therapies in India. It acts in a targeted manner at an early stage of the disease progression and is also an immunomodulator which treats the condition at an intracellular level whereas the other available drugs in the country are immunosuppressant. It is an oral therapy which can be self-administered unlike some of the currently available injectable therapies which have to be administered by paramedics. Further, Apremilast is a safer drug having no effects on other organs like the liver and kidney and does not require routine laboratory diagnostic tests like CBC, liver and Kidney test or TB screening as required in the case of other therapies used currently.
India has now become one of the largest patient pools in the world and is estimated to have around 33 million psoriasis patients. As per a study on psoriasis in India, based on data collected across various medical colleges located in Lucknow, Dibrugarh, Kolkata, Patna, Darbhanga, New Delhi and Amritsar. It was found that the incidence of psoriasis among total skin patients ranged between 0.44 and 2.2%. It was also found that the ratio of male to female (2.46:1) was very high and the highest incidence was noted in the age group of 20-39 years.
Want to write for InnoHEALTH? send us your article at magazine@innovatiocuris.com
Read all the issues of InnoHEALTH magazine:
InnoHEALTH Volume 1 Issue 1 (July to September 2016) – https://goo.gl/iWAwN2
InnoHEALTH Volume 1 Issue 2 (October to December 2016) – https://goo.gl/4GGMJz
InnoHEALTH Volume 2 Issue 1 (January to March 2017) – https://goo.gl/DEyKnw
InnoHEALTH Volume 2 Issue 2 (April to June 2017) – https://goo.gl/Nv3eev
InnoHEALTH Volume 2 Issue 3 (July to September 2017) – https://goo.gl/MCVjd6
InnoHEALTH Volume 2 Issue 4 (October to December 2017) – http://amzn.to/2B2UMLw
InnoHEALTH Volume 3 Issue 1 (January to March 2018) – https://goo.gl/fksdQx
Connect with InnovatioCuris on:
Facebook: https://www.facebook.com/innovatiocuris
Twitter: https://twitter.com/innovatiocuris
LinkedIn: https://www.linkedin.com/groups/7043791
Stay updated about IC, visit: www.innovatiocuris.com