Source: www.healthcareradius.in
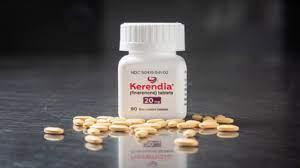
German pharmaceutical firm,Bayer has recently launched a potential blockbuster drug in India under the brandname ‘Kerendia’ to treat chronic kidney disease (CKD) in type2 diabetic patients. The drug is called Finerenone which is a first-in-class-non-steroidal, selective mineralocorticoid receptor antagonist.
The differentiating point of finerenone from the existing drugs for CKD in diabetic patients is that it works by blocking overactivation of the mineralcorticoid receptor (MR), which is thought to contribute to the progression of cardiovascular damage and chronic kidney disease.
This drug has been approved in India with an aim to lower the risk of an end-stage kidney disease, sustained estimated eGFR decline, non-fatal myocardial infarction, cardiovascular death and hospitalization for heart failure in adult patients with chronic kidney disease (CKD).
More than 13000 patients were enrolled in the phase III clinical trial program to explore the efficacy and safety outcomes, particularly cardiovascular and renal in patients with chronic kidney disease and type 2 diabetes. It was observed that Finerenone reduced kidney composite outcome by 23% on top of optimized Renin-angiotensin system (RAS) blockade and chance of a 57% Glomerular Filtration Rate (GFR). It also significantly lowered the risk of a composite CV outcome by 14%.