Source: www.Fda.Gov
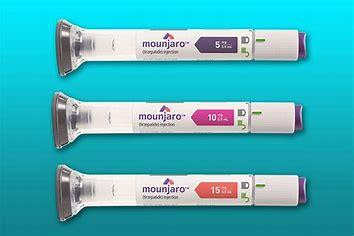
Type 2 diabetes is the most common form of diabetes which is a chronic, progressive condition in which the body does not make or use insulin normally, causing higher levels of glucose in the blood. It is estimated that more than 30 million Americans have type 2 diabetes, making 1 in 10 individuals a diabetic. The US FDA has approved a novel, dual-targeted treatment for type 2 diabetes ‘Mounjaro’ (tirzepatide) injection as an addition to diet and exercise.
This potential therapy is a once-weekly injectable dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide receptor agonist (GLP-1) which aims to control blood sugar. It is a subcutaneous injection wherein GLP-1 and GIP receptors cause the pancreas to release insulin and block the hormone glucagon thereby limiting blood sugar spikes after meals. This drug also slows digestion and helps diabetic patients to remain fuller for a longer duration and eat less.
Late phase 3 trials show that the treatment significantly reduces hemoglobin A1c in type 2 diabetic patients and aids in weight loss, potentially making it the most effective therapy for diabetes and obesity which has been developed till date.