Source: Pubmed
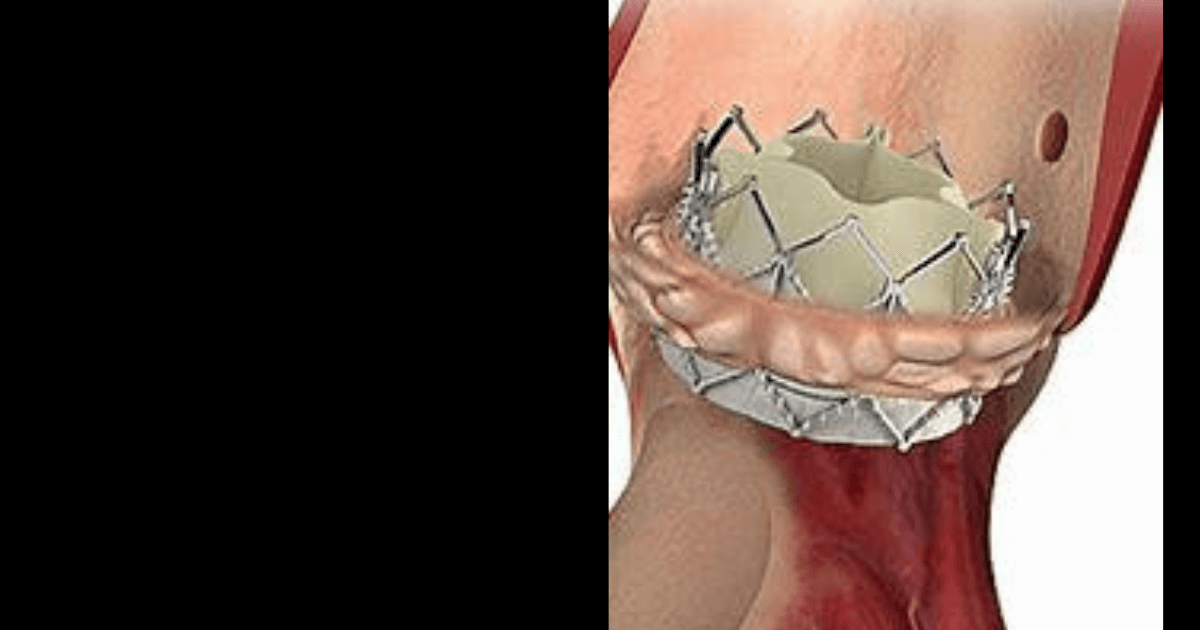
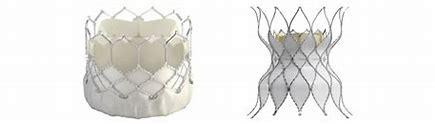
An American medical technology company, Edwards Lifesciences specializing in artificial heart valves and hemodynamic monitoring, has recently launched Sapien 3 transcatheter pulmonary valve system with Alterra adaptive pre-stent which is a catheter based stent and artificial heart valve for treating patients whose right ventricular outflow tract becomes too leaky.
The catheter-based stent, Alterra is made from self-expanding nickel-titanium and Sapien 3 artificial heart valve is made of cow tissue attached to a balloon-expandable, chromium-cobalt frame. This system has been approved for use in pulmonary valve replacement in cases where the patient’s pulmonary valve conduit or artificial pulmonary valve stops working properly. To begin the treatment using this FDA approved system, a doctor inserts a catheter through a large vein in the leg. The catheter has a compressed Alterra stent at the end and is pushed through the blood vessels until it reaches the pulmonary valve. Then the stent is released and anchors to a patient’s right ventricular outflow tract and Sapien 3 valve is engaged through a balloon catheter and pushed until it reaches the location of the Alterra stent.
Once the artificial valve is in place, it is expanded by a balloon and anchored inside the Alterra stent and works in a manner which is the same as the old valve by opening and closing like a door to force blood to flow the right direction.