Source: cnn health
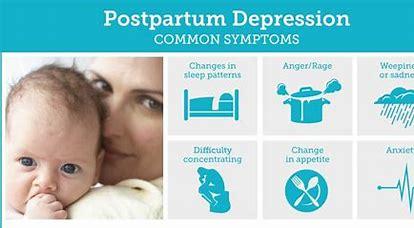

Postpartum depression (PPD)is a serious condition which, when severe, can be life-threatening and can interfere with the mother-infant bond. Because of being undiagnosed in most of the cases, experts believe that the prevalence of postpartum depression could be at least twice as high as what current statistics reveal. Primary treatment for PPD cases is counseling and antidepressants, but some women do not respond to these therapies, compelling more research in this area of medicine.
Recently, the US FDA approved “Zulresso” (brexanolene) injection for intravenous use for the treatment of PPD in adult women, making it the first drug approved by FDA for PPD. This novel drug has been approved with a Risk Evaluation and Mitigation Strategy which is available to patients through a restricted distribution program at certified healthcare facilities where the healthcare provider can carefully monitor the patient. This therapy, administered around the clock for 60 hours, uses a neurosteroid to control the responses of the brain to stress. This treatment targets the signaling, thought to be hormone-sensitive postpartum depression and also shows benefits very quickly in comparison to traditional antidepressants which typically take two to four weeks to display an effect.

